So what’s the big deal? We’ll just get some other company to take its place in the Dow Jones Industrial Average.
On Friday, a Texas jury held Merck & Co. liable for a quarter of a billion dollars for the death of Robert Ernst, a patient who had a heart attack while taking the drug Vioxx. The good news is that Texas law limiting punitive damages will cause this to be reduced at least 80%. The bad news is that Merck may end up forking over a comparable bundle to 50,000 other people in the U.S. alone; (Houston’s Clear Thinkers thinks fewer, but is still not cheered).
 |
The market seemed to shrug it off with only an 8% drop in the value of Merck stock. The reason for such a small move is that the stock instantaneously lost a quarter of its value last fall when Merck announced it was pulling the drug from the market and issuing a health advisory to users. Those capitalists, as I keep telling my readers, are forward looking and knew right then that lawsuits like the one in Texas were a-coming, and plenty of them.
But a quarter billion a pop? I took a look at one of the studies on which the decision was justified, written by Dr. David Graham and co-authors and published in Lancet in February. This study looked at 8,143 Kaiser Permanente patients who had suffered a heart attack and had also at some point taken a nonsteroidal anti-inflammatory drug (NSAID), of which Vioxx (rofecoxib) is one. Of these patients, 68 were taking rofecoxib while 4,658 were receiving no medication at the time of their heart attack, a ratio of (68/4658) = 1.46%. For comparison, the study looked at 31,496 other patients who had also at some point taken an NSAID, matched for characteristics like age and gender with the first group, but who didn’t have a heart attack. The ratio of rofecoxib users to those with no current medication was slightly lower (1.05%) in this second group, which one might summarize as a (1.46/1.05) = 1.39-fold increase risk of heart attack from taking rofecoxib compared to no NSAID. Is that statistically significant, in other words, can you rule out that you’d see a difference of that size just by chance? Yes, the study claimed, but just barely.
On the other hand, this was not a controlled experiment, in which you give the rofecoxib randomly to some patients and not others in order to see what happens. Rather, something about either these patients or their doctors led some of them to be using rofecoxib and others not. Dr. Graham and co-authors looked at a variety of indicators that suggested that the rofecoxib patients already had slightly elevated risk factors for coronary heart disease. Once they controlled for these with a logistic regression, their study found an elevated risk factor of heart attack for rofecoxib takers of 1.34, which was not statistically significantly distinguishable from 1.0.
The strongest evidence from this study was a claimed dose-effect relation. Of these 68 rofecoxib-using heart-attack patients, 10 of them were taking doses above 25 mg per day. Only 8 patients in the much larger control group were taking so high a dose, implying an elevated risk factor of 5 to 1 for high-dose patients. Again observable risk factors could explain some of this, with the conditional logistic regression analysis bringing the implied drug-induced risk down to 3 to 1. According to the study, this elevated risk factor was still statistically significant, even though the inference is based on the experience of just 10 patients.
The obvious question here is whether in fact the authors were able to observe all the relevant risk factors. The study openly acknowledged that it did not, missing such important information as smoking and family history of myocardial infarction.
So how do you explain these issues to a Texas jury? Well, the defendants argued these 5 to 1 odds aren’t statistically significant, to which the plaintiff’s lawyer Mark Lanier offered this folksy rejoinder (hat tip: Newmark’s Door):
Have you got $6 on you? I’m going to give you a dollar and you give me the six. It is not statistically significant in the difference. What do you think, are you in or out?
Sure, Mr. Lanier, I’m in, though here’s how the deal actually works– you give me the dollar, but you don’t know whether I give you $6 or I give you nothing. Or, to be a little more accurate, even if there actually is an elevated risk of the magnitude the studies suggest but can’t prove, the question is whether I might want to accept a 1 in 4,000 risk of dying from a heart attack in order to get the only medication that makes my pain bearable and a mobile life livable. And if I say no to the Vioxx, I may end up taking something that is less effective for my pain but has risks of its own.
And now I have a question for Mr. Lanier. How did we arrive at a system in which 12 random Texans are assigned responsibility for evaluating the scientific merits of statistical evidence of this type, weighing the costs and benefits, and potentially sending a productive blue-chip American company into bankruptcy protection?
Evaluating the true risk of Vioxx
At the start of the recent Merck/Vioxx trial, this post noted the dearth of clinical evidence that Vioxx was a particularly risky drug. In light of last week’s big verdict in the case, long-time Clear Thinkers favorite James D. Hamilton…
Smug Superiority Complex
Econbrowser: Murky future for Merck (Via Tom K) Two points to highlight in a seemingly articulate post on the dangers that Merck now faces due to the $26M liability the firm now has from a court case. Point the first…
The Case Against “The Case Against Merck”
James Hamilton slams the prosecution:I took a look at one of the studies on which the decision was justified, written by Dr. David Graham and co-authors and published in Lancet in February. This study looked at 8,143 Kaiser Permanente patients
JDH: We arrived at the system of 12 jurors similar to the way we arrived at the system that allows the American public to elect an idiot like George Bush who costs the American tax payers over $200 billion for an unnecessary war.
And the problem with the argument about all the prescient forward looking capitalists is that these were the same ones who overpriced stocks to the tune of several trillion dollars before the bubble burst.
This is not an argument against capitalism. Nor free markets. I’m all for them. It is an argument for context and perspective.
I’d like to see a more rational process by which corporate responsibility is levied. But I’d also like to see a more rational process by which we select leaders. In the mean time, since I’m not holding my breath and certainly am not covinced that there are arguably superior selection techniques, I’ll look to the big picture: The threat of lawsuits has changed the way companies think and operate, they focus more on safety, they focus more on public perception, and I’m not sure the overall cost–negative or positive–is well represented by a particular case like Merck. Economists can always data mine and selectively pull out results to “prove” their points, but I still argue that I’ve not seen much to indicate to me that economics is much of a predictive nor explanatory science for all but the essentials like monetary theory. That doesn’t mean we shouldn’t keep trying, but I’m always concerned that the murky nature of economics simply allows economists to paint their ideological predilections with language that sounds irrefutable–not that disimilar to our friend Mr. Lanier.
Lying with Statistics – Ernst v. Merck edition, Part II
Econbrowser’s James Hamilton takes a detailed statistical look at the Graham et al. Lancet Vioxx study that was the subject of the now infamous cross-examination of Merck’s CEO, where Mark Lanier argued (apparently successfully) that the concept of “st…
::::
Economists can always data mine and selectively pull out results to “prove” their points, but I still argue that I’ve not seen much to indicate to me that economics is much of a predictive nor explanatory science for all but the essentials like monetary theory.
::::
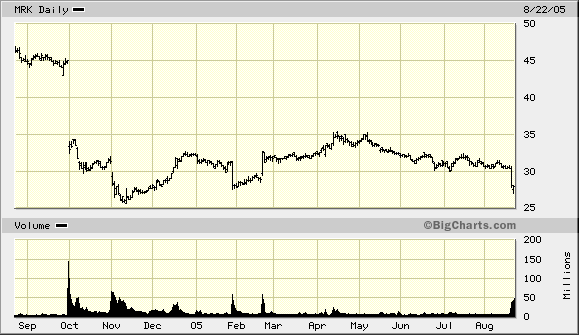
True, since economists are often studying phenomena that are interesting but beyond experimental control, data mining is a very real danger (our blog host likely agrees). However, is it really the case with the graph above that basic economic theory hasn’t helped us understand what happened in October? The tech stock bubble burst, but that was a case where many people were investing in assets more risky than was advertised at the time: it makes sense that as realizations of those correlated random events started to happen, people would update the riskiness of their positions and find their portfolios unattractive and sell.
But with the graph above, it is a clean example of how information about the future was incorporated into prices. I think anyone who has calculated the discounted present value of something should find that graph pretty cool.
Professor,
I agree with you that Merck had at least a decent case based on science alone. But according to the WSJ, what incensed the jurors, is that Merck covered up the heart attack risk from Vioxx that it aleady knew about. Merck got slammed for a lack of honesty as much as anything else. And well they should have been.
I have more than a passing interest in this one, because my wife, who has persistent chronic pain, uses Celebrex under doctor’s prescription. In her case, taking Celebrex is a no-brainer, given the small but significant cardiovascular risks — she needs the prescription in order to function and go to work. Yet because her doctor can monitor her closely for heart risk she is willing to continue on Celebrex. Had the risks been concealed from us, you can bet we would be outraged.
As Professor Friedman wrote in Capitalism and Freedom, the government’s job is to prevent force and fraud so that free markets can function efficiently. By marketing Vioxx as a riskless drug without side effects, Merck engaged in fraudulent conduct that is inimical to the proper functioning of free markets.
SP: One side point: Don’t get me wrong. I think economics is both fascinating and an important means to creating a more rational free society, etc. etc.
As far as the graph: I agree that it shows reality hitting investors in the face when a major drug was pulled and the threat of lawsuits increased. But the question remains: is the stock rationally priced now? Are investors telling us something about the future price of the stock based upon todays price? Does the minimal movement in the past few days mean something? I think the honest answer is: nobody really knows. Or as Jim Morrison once said: “The future’s uncertain and the end is always near.” Perhaps he was referring to Merck? Nah. Probably himself. 🙂
“I agree with you that Merck had at least a decent case based on science alone. But according to the WSJ, what incensed the jurors, is that Merck covered up the heart attack risk from Vioxx that it aleady knew about. Merck got slammed for a lack of honesty as much as anything else. And well they should have been.”
On the science alone, it isn’t clear there was anything to warn about. If a scientist sends an email that says, “I think we should look into this” is it really time for a press release? This decision incents the drug companies to do less, not more, research into the harmfulness of their products. The word is probably going down from on high in the surviving drug companies that no one is to put into writing any concerns they have about the products because internal discussions about risks are too confusing to juries.
Dear Anon,
It was alot more than “we should look into this.” In fact, for example, written records showed that when Merck sales reps met with doctors, the reps were specifically instructed how to play a game that memos called “dodgeball. ” According to the rules of “dodgeball,” the reps would deflect and conceal the information the company had regarding Vioxx risks in response to doctors’ questions. This concealed information included data from large double-blind studies, which again, was a heck of a lot more than a “we should look into this.”
For the system to work, we need patients to be able to trust their doctors to provide accurate information, and we need doctors to be able to obtain that information freely.
Jonathan
::::
But with the graph above, it is a clean example of how information about the future was incorporated into prices.
::::
I misspoke and you are right, T.R.: its not the future that is put into the price — no one knows the future.
What I should have said is that the graph above is a clean example of how information about the probabilities of future events was incorporated into prices.
So I think that today’s price tells us something about how investors consider the probabilities of relevant future events (along the lines JDH has pointed to when discussing oil futures in past posts).
To the extent that investors know something about the uncertain movement of the future value of an asset, their changing valuation should say *something* about the probabilities (from their POV) of possible future values. If investors put their subjective probabilities to work to determine what value they think the stock has today and then bought or sold accordingly, wouldn’t that be rational? Or do you think that these subjective probabilities need to line up (however loosely) with some standard probability distribution in order to be considered rational?
I suppose I’m asking, are speculative bubbles irrational?
Let’s say we have existing and commonly accepted drug A, and new drug V. They have the exact same positive effects, with this change in side effects per 50,000 people:
Drug A V
—————————————–
Fatal Heart Attacks 100 10
Head Explodes 0 1
It doesn’t matter that for every 50,000 people taking the drug, 89 lives will be saved. The plantiff’s lawyer will get up before the jury and excorciate drug V’s manufacturer for playing God and trading lives. Even better if they can dig up internal memos from company V showing that “the 1 head explosion is worth the risk” — juries HATE IT when confronted with the risks of the every day world.
Want even more fun? Pretend there were now two existing drugs, A and B, with these risk profiles, as compared to V:
Drug A B V
———————————————-
Fatal Heart Attacks 100 0 10
Fatal Stroke 0 100 10
Head Explodes 0 1
Someone switches from drug B to drug V and dies of a heart attack. Well, it’s obvious that drug V caused the heart attack, so it’s time for them to pay up.
In a rational society, V’s manufacturer would make up for the increased safety through a better price on the drug, and could cover the smaller loss of life through the increased earnings. But with punitive damages, V’s manufacturer has to pay at a high multiple of the number of lives lost.
JDH can you please explain the stastical numbers viz. 8146 and 31,496 more lucidly. I am not able to figure out how they are relevant.
In a simple world securities valuation would indeed represent the value of the benefits of ownership (dividends and terminal value assumptions) factored by the perceived risk of the investment. However, many large investment managers (mutual funds and pension funds) are either prohibited by their charter from holding “high risk” investments, or are want to report that they have investments in companies with recent bad news.
Bad news can obviously presage the total elimination of value (Enron, Worldcom, etal.), and thus there are always some winners in a sell, sell, sell mentality. However, there are many other Companies that have survived bad news and the market almost always moves to a short term extreme.
Markets are indeed rational over an extended period of time, but are incredibly emotional in the instant. Either today’s sellers, or today’s buyers will be wrong as to what they thought the stock would be worth tomorrow. One-half of the people are always wrong, and one-half are always below average.
Bill
“the question is whether I might want to accept a 1 in 4,000 risk of dying from a heart attack in order to get the only medication that makes my pain bearable and a mobile life livable.”
Well this gets to the heart of the matter.
All Merck had to do was make the data it was aware of available to medical professionals so that the risk/benefit ratio of the drug could be properly evaluated by them, & so they could, in turn, advise and consult with the patient on the appropriate therapy regiment.
But they didn’t. They hid the data.
So, now….?
.
Atanas, all the patients in the study had taken NSAID at some point, some currently as of the date reported, others previously, some rofecoxib, most alternative NSAID. 8,143 is the number who had heart attacks (“cases”). 31,496 is the number who did not have heart attacks, but who were selected to be similar to the first group (“controls”). The study’s conclusions are based on how the drug experiences of these two groups differ.
Of the cases, 68 were taking rofecoxib at the time of the heart attack. Of the controls, 196 were taking rofecoxib at the time of their observation. A simple thing you might have considered doing is look at the fraction of cases on rofecoxib (68/8143) relative to the fraction of controls on rofecoxib (196/31496), which ratio is 1.34. So there are a little more rofecoxib users among the people who had heart attacks, which would lead you to think that rofecoxib may have raised the risk of a heart attack. What the study instead did is to look at the ratio of rofecoxib to currently no drug in the cases (68/4658) relative to the corresponding ratio for the controls (196/18720), which ratio is 1.39. The two ratios give slightly different answers (1.34 instead of 1.39) because not only was there a little more rofecoxib use among the cases, there was also a little fewer people taking no drug. Coincidentally, 1.34 is also the number that the 1.39 ratio becomes once the study controlled for observable cardiac risk variables.
I would like to see a more libertarian attitude towards consumer products in which all information is provided up front and consumers are then allowed to make the choice. How risky, for example, is a particulare pain medication when compared with driving a motorcycle in Los Angeles.
This then raises the question of whether, for example, automakers would have focused on safety at all. Would consumers have had a choice? Is Govt regulation the solution? Threat of lawsuits.
It is choice that should be maximized. I suppose the primary problem is that choices are often not available, not just that informed choices are not available, but that automobiles with air bags and/or safety belts aren’t available at all without the threat of regulation and/or lawsuit.
Merck and the Vioxx Decision
James Hamilton has a very nice post on the dubious nature of this decision. Hamilton walks us through some of the statistical arguments and concludes the following,
So how do you explain these issues to a Texas jury? Well, the defendants argued the…
“And now I have a question for Mr. Lanier. How did we arrive at a system in which 12 random Texans are assigned responsibility for evaluating the scientific merits of statistical evidence of this type…?”
I don’t know exactly how they do jury selection in Texas, but I doubt it’s random. My question would be for Merck’s attorneys: Why couldn’t they get a better jury? If there had been one single person on the jury with a basic understanding of statistics, I’m sure he or she could have convinced more than one other person to find for the defendant. (The decision was 10 to 2.) Merck’s attorneys either dropped the ball or were cheated or got very unlucky on jury selection.
They also should have advised Merck (perhaps they did) never to do anything that looks like a cover-up. Indeed, if Merck’s executives read the newspapers, they should have known that even without their attorneys’ advice. It is a shame if this brings down the company, but they really should have known better.
I looked at editorials in major medical journals, and found that none of them shared Hamilton’s scepticism about Vioxx’s harmfulness. For example, the editors of the Lancet say ‘the licensing of Vioxx and its continued use in the face of unambiguous evidence of harm have been public-health catastrophes.”‘
I think these experts disagree with Hamilton because there are many studies that find that Vioxx causes heart attacks, including one randomized trial, not just the one he discusses.
Also, Hamilton’s claim of a 1 in 4,000 chance of death from Vioxx is misleading at best. Most studies look at the increased risk of having a heart attack, not dying from one. And they find much higher figures for the increase in heart attacks — more like 1 in 100.
Since not everybody who gets a heart attack dies, estimates of the chance of death are going to be based on quite small samples, and not tell us much of anything. I’d like to see a confidence interval on Hamilton’s 1 in 4,000!
I discuss this further on my blog:
http://ragout.blogspot.com/2005/08/evidence-against-vioxx.html
Good points Ragout.
I would only add that the analogies being made between the pharmaceutical market and other consumer markets are, at best, specious.
The consumer does not decide which medication they will be given. The consumer’s physician decides (with some important input from the consumers insurance company’s drug formulary).
One analyst claimed that the contigent liability from these lawsuits is $50 billion, which would be over 80% of the value of the company. It seems the market does not agree.
Jonathon wrote:
“…according to the WSJ, what incensed the jurors, is that Merck covered up the heart attack risk from Vioxx that it aleady knew about. Merck got slammed for a lack of honesty as much as anything else. And well they should have been.”
I agree Merck should be slammed for lack of honesty, but no “slamming” should be allowed in a court of law if the lack of honesty had nothing do with causing plaintiff’s injury.
One has to believe that if Merck had only issued an appropriate warning of an elevated risk factor of 5 to 1 for high-dose patients (no evidence plaintiff fell into this category), the warning would have made its way immediately to doctors, including plaintiff’s doctor, who would have immediately contacted all patients for which VIOXX was prescribed. Once contacted, plaintiff, now knowing of the increased risk to high-dose patients (which he was not) would have thoughtfully weighed the new risks, however slight, ceased using the drug and would be running marathons to this day day (presumably either in great pain, or with the use of the multitudes of other completely risk free economically feasible alternatives to VIOXX)
Pharmacuetical ads today are almost akin to SNL skits: “side effects may include balding, diarrhea, boils, hallucinations, and the occasional exploding head..” Plaintiff: “If only the drug company was more honest about the 1 in 1,000 chance of my toe nails turning yellow, I would have opted not to use this medication. A quarter of billion dollars payable to me and my attorney should send a message!”
now choose t
If the makers of Celebrex conceal useful information from you and your wife
http://www.janegalt.net/blog/archives/005426.html
Great post on the statistical signifigance of the Vioxx results from Econbrowser. Frankly, given how low the relative increase in risk is, I don’t understand why patients aren’t being given the option of taking Vioxx after being rigorously informed of …
Great post. I fail to see how the rest of us gain by having a possible link of Vioxx to a portion of certain deaths and injuries turn into the death penalty for a drug company.
We are witnessing the slow dissolution of medicine in the US. Attacked by third party payors, government regulators, Medicare, Medicaid, and lawsuits, medical care is going to suffer. Its already difficult to find a doctor willing to deliver a baby, a neurosurgeon willing to care for head injury, or dentists willing to see the poor. Heck, one MD got reprimanded by the NH Medical Board for telling a fat woman she was obese.
Vaccines are scarce in no small part due to the effect of numerous lawsuits in the 1970s. Certain drugs will be deemed by their manufacturers as ‘too risky’ from a legal standpoint, and instead of hiding the evidence, they’ll simply withdraw the drug.
Incentives matter. The incentives in health care are tilted towards staying away from the product and service end.
The pharmaceutical industry makes billions of dollars drugging school children and this is a form of genocide: condemning millions of young lives to a drug addicted future. They employ experts and lobbyist and hire ex FDA personnel and retired congressman to get pro-drug legislation passed. Newspapers and magazines receive billions of dollars a year in advertising, and investment firms make big bucks touting the latest snake oil; so it would be a rare article indeed that went against Big Pharma. The industry is motivated by the bottom line and shareholders not Science. A Google search of Ritalin and Cocaine, Prozac, chemical imbalance, school shootings, will show even the most skeptical that something is horribly wrong when 6 million school children ( plans are in place to increase this by 40% each year) are on anti-depressant drugs prescribed to handle disorders created to sell the drugs. Now after the Texas Vioxx decision Big Pharma’s stooges are flooding their editorial outlets ( USA Today, the New York Times, Wall Street Journal)with demands that the government protect the drug companies. Michael
How Dangerous Is Vioxx?
Vioxx has been removed from the market because it has been linked to an increased chance of cardiac events. But, how much extra risk is there? That sort of information is harder to come by. Here is what I constructed
This result demonstrates that the adoption of the Daubert rules by states is not foolproof (Texas has adopted the Daubert rules of evidence). In this case there appears to be a dispute about statistical significance. Typically, when confused about a scientific issue, the jury will essentially disregard it and look for easy to understand issues on which to render a judgement that may be irrelevant to the actual dispute. That appears to be what has happened here.
Naturally, there will be an appeal and then some sort of settlement that will pay everyone off. The only question is, how much the payoff will be.
Michael:
The “pharmacetical industry” has not drugged my children – nor would I allow it. Further, victims of genocide do not normally line up and pay for the service.
Advertising and lobbying may help sell and expand markets, but it did not create demand for these drugs. The combination of redefining what is considered appropriate adolescent male behavior and an abdictation of parental and educational responsibilty for discipline has resulted in the creation of these previously unknown or unrecognized “disorders”.
I agree lobbying will assist in redifining certain behavioral and other problems as “disorders” or even “diseases” (alcoholism). Once defined as a disorder or disease, the health care insurers become hard pressed to deny benefits for prescriptions to treat the newly created malady. What irks me is that even though I will never avail myself of these “snake oils”, I am either forced to fund it, or go uninsured.
Yes, American juries have no understanding of statistics or multivariate causation. The question is whether a panel of judges is better(judges are selected by politicians based mostly on political factors- thats why the Texas appellate courts are full of Republicans. They wont neglect Mercks interests) However, Hamilton is flat wrong to use Grahams observational study from the Lancet, when better data (i.e. large randomized controlled trial) is available..
I am an academic physician; randomized controlled trials provide the best way to estimate the difference between two treatments. This is because patients are randomized between the two treatments so that differences not due to the drug should equal out- observational trials like the Graham study are misleading because we doctors dont give the same drugs to every patient with a particular disease.
For rofecoxib (Vioxx) we must review the large VIGOR trial
1. The VIGOR study included 8076 patients with rheumatoid arthritis randomized between naproxen and rofecoxib. The relative risk of myocardial infarction between naproxen and rofecoxib was 0.2. Let me quote from the summary in 2000 in the New England Journal of Medicine (Bombardier, et al). The incidence of myocardial infarction was lower among patients in the naproxen group than among those in the rofecoxib group (0.1 percent vs. 0.4 percent; relative risk, 0.2; 95 percent confidence interval, 0.1 to 0.7). Five to one- thats large; most internists and cardiologists knew this by 2002 because of agitation by people like Dr. Topol from the Cleveland clinic.
2. You can access FDA rofecoxib data on the Internet although the drug was approved by the FDA in 1999, the Arthritis Advisory Committee didnt meet to review rofecoxib cardiovascular risks rofecoxib until August 2001. The FDA considered sudden cardiac death without myocardial infarction, myocardial infarction or the development of angina pectoris to be serious cardiovascular events- there was a great excess of these events in the rofecoxib group. The committee concluded that “it is mandatory to conduct a trial specifically assessing cardiovascular risk and benefit of these agents.” No such study was ever done, and the main users of Vioxx were older people with chronic arthritis, a group with an increased risk of myocardial infarction even without Vioxx rheumatoid arthritis is an inflammatory disorder, associated with increased CRP and greater risk of stroke and MI.
3. A detailed review of the VIGOR trial allows one to conclude that there are a few patients who probably would do better with rofecoxib- those with known high risk of GI bleeding and known low risk of heart attack. That point is made by Choi et al. However, that is a small niche, whereas the Dorothy Hamill ads and the abysmal behavior of most American physicians resulted in Vioxx prescriptions for many people at high risk of stroke and heart attack, many of whom never tried the safer conventional NSAIDs. How could a physician read that 5 fold greater risk of myocardial infarction and blithely keep prescribing it? Obviously, many doctors didnt want to be bothered with the facts. I know several physicians and physicians wives who bought hundreds of Vioxx tablets last September when the drug was withdrawn- they swear that it is a miracle drug. I have been subject to bouts of nasty back pain from time to time. I had one in 2002; my physician, an internist, advised me to take Vioxx. I told her, no way, that drug increases the risk of thrombosis and I know that I can tolerate aspirin. I took moderately high aspirin doses for 2 weeks and did well. My physician knew about the VIGOR trial and said, but if you take less than 50 mg/day the risk should be less and its a once a day drug. She was correct- the risk of adverse effects depends upon dose and the duration of use and once a day medication is more convenient. However, old people with osteoarthritis generally tend to keep taking a drug indefinitely, which is clearly unwise even for Ibuprofen or Naproxen.
Here are my references. I can email pdfs of these papers to Prof Hamilton or anyone wanting to read them. I like this blog but Hamilton struck out this time. The Texas man who died from cardiac arrhythmia and only possible MI would have been considered a serious cardiovascular event by the FDA but not by Mercks man Bombardier- read the paper.
Bombardier, C, et al, Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 2000; 343:1520-8.
Choi, HK, et al, Effects of rofecoxib and naproxen on life expectancy among patients with rheumatoid arthritis: a decision analysis. Am J Med. 2004; 116:621-9.
Topol EJ. Failing the public health: rofecoxib, Merck, and the FDA. N Engl J Med. 2004; 351:1707-1710.
Bob Snodgrass, Torrance, CA
Dr. Snodgrass,
I would add that the APPROVE study, another randomized trial, also found that Vioxx raises the risk of heart attacks. It was this study that Merck cited as prompting their decision to pull Vioxx from the market. As I said above, by reading editorials in major medical journals the layperson can easily verify that there’s no serious doubt that Vioxx causes heart attacks. (see my blog for references).
I have one question. Given that the VIGOR study in 2000 raised serious concerns about Vioxx-related heart attacks, why were doctors so quick to prescribe it? Why did medical journals continue to advocate its “first-line” use (see reference below)?
It seems to me that everyone failed on this one: Merck, the FDA, academic physicians, practicing doctors.
Lanas A., Clinical experience with cyclooxygenase-2 inhibitors.
Rheumatology (Oxford). 2002 Apr;41 Supp 1:16-22; discussion 35-42.
The APPROVe trial used a lower dose of Vioxx or rofecoxib but enrolled people of similar age to the VIGOR study in a controlled trial, just fewer of them. The study showed (Table 2 of Bresalier et al) that serious cardiac events (not just MI) were 2.8 times more frequent in people getting rofecoxib hoping to reduce recurrence of colonic polyps than in those taking an inert placebo. That is a smaller difference than found in the Bombardier paper- the dose was less and it is possible that there are beneficial effects of Naproxen (we are always told don’t give it to people with Von Willebrand disease or recovered ITP because of its antiplatelet effects. Still a 280% increase in the risk of serious cardiac events should frighten most patients, and gives a different and more valid picture than the Graham paper.
Who failed? Almost every body, Merck most of all. Most American physicians want to get their medical information and a free lunch from a drug rep. This is not good, but when drug companies finance so much of medical education that’s not surprising- drug reps are chosen to be very personable and their free lunches are painless. Many academic docs were very enthusiastic about selective COX-2 inhibitors when they were in the developmental stage- Jerome Groopman wrote a cheerleader piece in the New Yorker, superaspirin, but the concept sounded good. We keep hoping for miracle drugs.
Bob Snodgrass
Dr. Snodgrass and Ragout, thanks much for your highly informed and informative comments. I believe that Dr. Snodgrass’s judgment that “observational trials like the Graham study are misleading because we doctors don’t give the same drugs to every patient with a particular disease” is precisely the same point that I was making when I said “something about either these patients or their doctors led some of them to be using rofecoxib and others not.” Dr. Snodgrass appears to be skeptical of this study, indeed dismissing it altogether, for exactly the reason that I gave in my post as one of the arguments for being skeptical of this study.
While we appear to all agree that this particular study is unconvincing, Dr. Snodgrass and Ragout criticize me for only talking about this one study. I apologize if I gave readers the impression that I had exhaustively analyzed every study of Vioxx. What I did was to pick one study that seemed to be getting a lot of media coverage and that appeared in what I perceived to be one of the most prestigious medical journals, and looked at it from the point of view of how convincing was its statistical conclusion on the basis of the evidence that the article presented. I was thinking of it as an exercise of using my training in statistics to examine in detail one part of the picture that was being covered in a fairly cursory manner by much of the media. I only looked at one study because that was all I had time for. I was wrong to fail to communicate that what I was doing was looking at one piece of evidence in detail as opposed to evaluating all of the evidence that anybody has presented.
I nevertheless stand by my statements that: (1) the evidence in this particular study fails to prove a connection between Vioxx and heart attacks; (2) it is a highly problematic system to ask a jury of 12 lay people to evaluate the scientific merits of this kind of evidence; (3) our legal tort system is not constructed to generate a sound evaluation of costs and benefits in cases like this one.
“I fail to see how the rest of us gain by having a possible link of Vioxx…”
Howza’bout: “it’ll make the whole bunch of ’em think twice before they try ‘n pull a stunt like hidin’ data again”
Then you (and your family) can take medications prescribed by you physician whithout wondering if the pharmaceutical industry just thinks of you as another test animal in some (post marketing) clinical trial.
There’s an interesting post on some of the Vioxx evidence here: http://www.journalclub.org/2005/01/25/n50
A followup on my comments from 25 August. In a today’s Washington Post, an article titled VIOXX HEX has this gem:
Unfortunately for Merck, scientific facts didn’t play much of a role in the first Vioxx trial, which ended on Aug. 19. The Texas jury in that case awarded $253.4 million to the widow of a man who died of a heart attack triggered by arrhythmia, which is not a condition Vioxx has been proven to cause. The jury, declaring that it wished to “send a message” to Merck, decided to make an enormous symbolic award anyway. Besides, said one juror afterward, the medical evidence was confusing: “We didn’t know what the heck they were talking about.”
Q.E.D.
Of course the study showed that serious cardiac events were 2.8 times more frequent in people getting rofecoxib hoping to reduce recurrence of colonic polyps than in those taking an inert placebo.
The healthcare system would be so much more efficient if there was free market competition between healthcare providers.