Really, you read “The Hill” article, you literally don’t know anything.
According to a former federal trade official, enforcing this sort of directive will not be simple, especially the provision requiring facility development in the U.S.
“How on earth do you enforce that? How do you make sure that that is clear at the time of import? Even more so, [Harmonized Tariff Schedule] codes don’t distinguish between brand and generic,” Monica Gorman, managing director at Crowell Global Advisors and former special assistant to former President Biden for manufacturing & industrial policy, told The Hill.
“Enforcing this is going to be incredibly challenging, but at least from an immediate impact standpoint, the fact that it appears to exclude generics is incredibly important and is likely to lessen the impact on the American patients,” she added.
It remains unclear whether this order was informed by the Section 232 investigation ordered by the Trump administration.
Earlier this year, the Commerce Department initiated an investigation into the national security impact of importing pharmaceuticals and pharmaceutical ingredients. This was done under the Section 232 of the Trade Expansion Act of 1962, which gives the president authority to restrict imports that may threaten national security.
If this action was spurred by the 232 investigation, then the tariffs may be stronger as a result.
…
[emphasis added]
Open question: Don’t we need a report released before Section 232 tariffs are imposed?
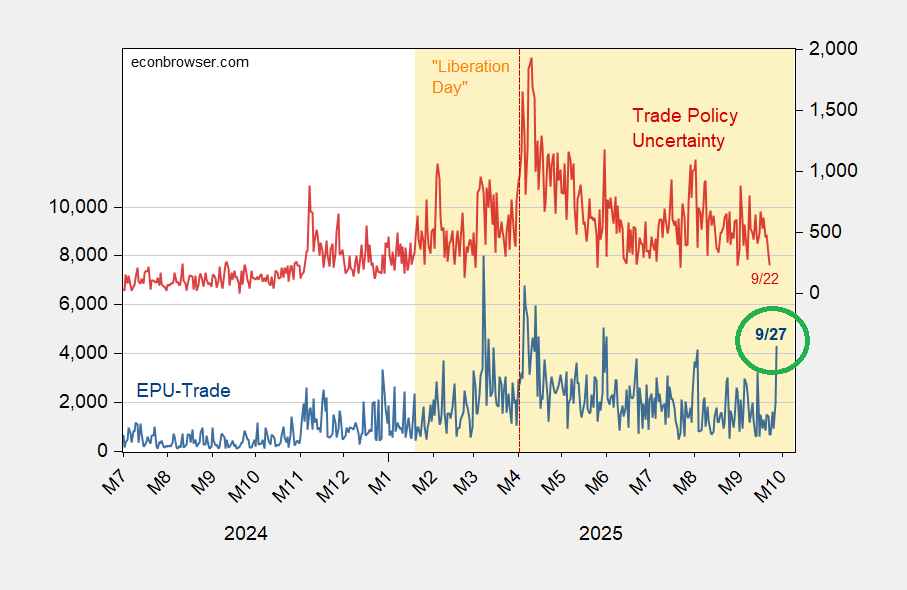
Figure 1: EPU-trade category (left scale, blue), Trade Policy Uncertainty Index (red, right scale). Source: policyuncertainty.com, matteoiacoviello.com.
Here’s a backgrounder on generics as a share of the global pharmaceuticals market:
https://www.statista.com/topics/12975/generics-and-biosimilars/#topicOverview
Brand name drugs are by far the larger segment, based on expense. Generics are by far the larger segment, based on volume. You can figure out what that implies about relative prices, and U.S. consumers pay the highest price of all, by design.
Trump is bullying the drug companies to reduce drug prices in the US.